This is the second post in a series on inositol supplementation. The first post provided an overview of what inositol is. If you haven’t read that one, go check it out first!
This post goes into detail on several studies that demonstrate the effectiveness of inositol supplementation for improving reproductive hormone levels, insulin resistance, and cardiovascular health.
Females with polycystic ovary syndrome (PCOS) almost always have underlying insulin resistance as a root cause of their symptoms and are at higher risk for cardiovascular disease, so any supplement that can improve markers for metabolic and cardiovascular health among females with PCOS deserves discussion.
It should be noted that there are more studies than those discussed here, but I have chosen to highlight several studies that were designed using scientifically sound methods and that are representative of the broader results found.
supplementation with d-chiro-inositol alone for pcos
The first study to test the efficacy of any form of inositol for PCOS focused on d-chiro-inositol (Nestler et al. 1999). This study recruited 44 females with PCOS and BMI’s greater than 28 (a BMI in the middle of the overweight range) from the Hospital de Clinicas Caracas in Caracas, Venezuela. Participants were randomly assigned to one of two groups:
Treatment Group: consumed 1.2 grams of d-chiro-inositol per day
Control Group: consumed 1.2 grams of placebo per day
Both groups consumed their respective substance for 6 to 8 weeks.
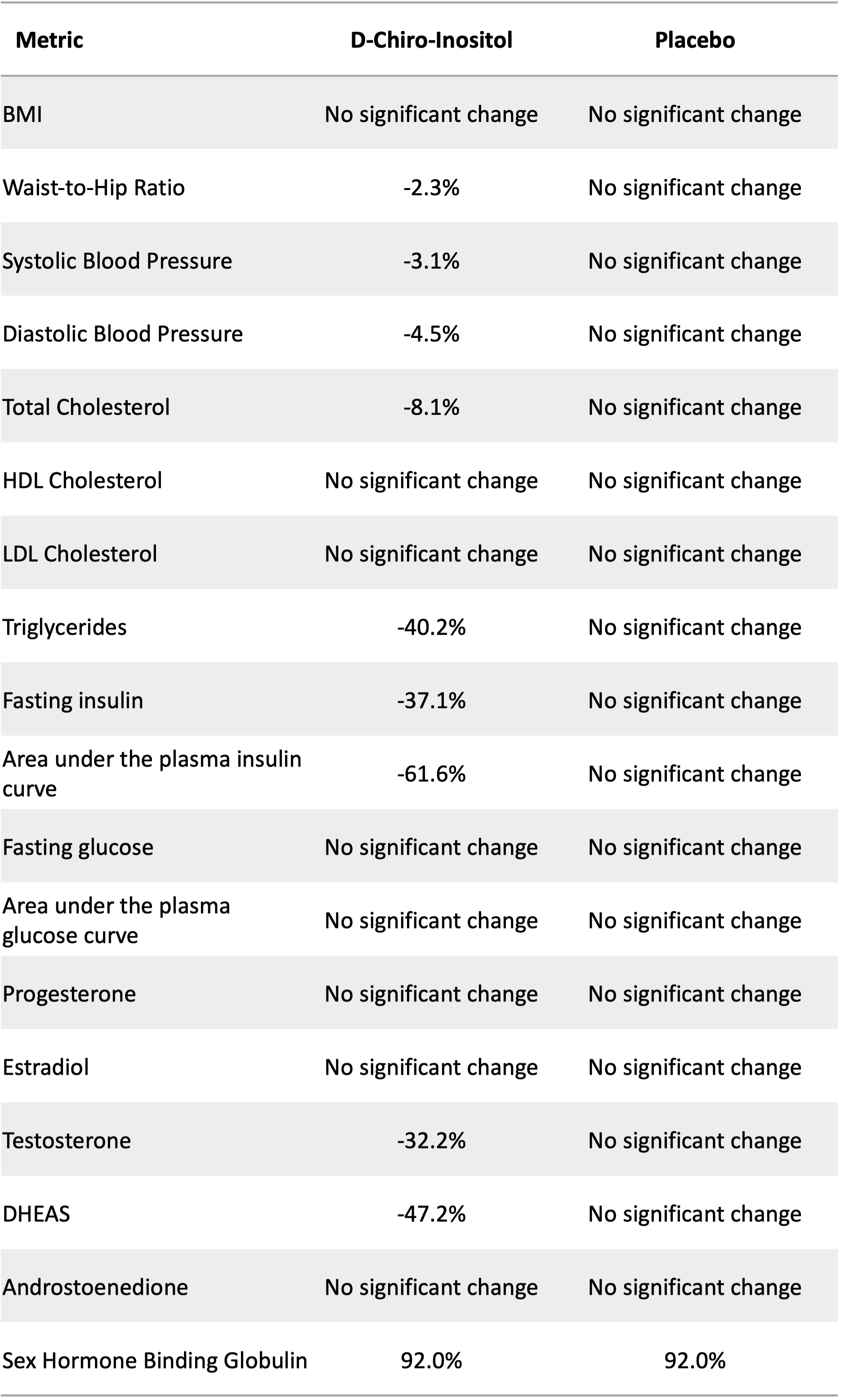
The table below summarizes the changes found after the treatment period. As we might expect, the placebo group experienced no significant changes to any measure.
The treatment group experienced modest improvements in waist-to-hip ratio (an indicator of metabolic health) and both systolic and diastolic blood pressure (top and bottom numbers, respectively, in a standard blood pressure measurement). The treatment group also experienced an 8% reduction in total cholesterol and a 40% reduction in triglyceride levels. The latter measure is a good indicator of improved cardiovascular health after supplementation.
In terms of other metabolic health markers, fasting insulin decreased 37% and the area under the insulin curve decreased about 62%. This latter measure tracks insulin response over time after consuming a glucose-based beverage. A smaller number indicates that less insulin was released in response to consumption of the glucose and/or the body cleared the insulin faster. Both indicate improved metabolic health.
Interestingly, even though the treatment group experienced improvements in insulin measures, they saw no improvements in the two blood glucose measures.
In terms of reproductive hormones, the treatment group experienced reductions in the two tested androgen hormones: testosterone and DHEAS. Females with PCOS tend to have elevated androgen hormones which contribute to acne, excessive body hair, and male pattern baldness. Treatment group participants also experienced a 92% increase in sex hormone binding globulin, which binds to hormones like testosterone, further reducing the androgenic effects of the hormone.
Percent change in body composition, cardiovascular health measures, metabolic health measures, and reproductive hormones after 6 to 8 weeks of supplementation with either 1.2 grams of d-chiro-inositol or a placebo by participants with polycystic ovary syndrome (PCOS). “No significant change” means that any differences found were not statistically significantly different than zero. Source: Nestler et al. (1999)
While the study by Nestler et al. (1999) discussed above restricted their sample to overweight and obese females with PCOS, Iuorno et al. (2002) repeated the experiment with females in the “normal” BMI category (20 - 24.4). Twenty lean females with PCOS were recruited from the same hospital in Caracas, Venezuela.
Participants were randomly assigned to one of two groups:
Treatment Group: consumed 600 milligrams of d-chiro-inositol per day
Control Group: consumed 600 milligrams of placebo per day
Note that this study used half the dose used in Nestler et al. (1999).
Both groups consumed their respective substance for 6 to 8 weeks.
The table below summarizes the changes found after the treatment period. While the placebo group experienced increases in blood pressure and total cholesterol, the treatment group experienced decreases. Similarly, the treatment group experienced a 52% reduction in triglycerides while the placebo group experienced only an 11% reduction.
In terms of markers of metabolic health, while the treatment group experienced reductions in both fasting glucose and fasting insulin levels, there was not a statistically significant difference between the changes experienced by the treatment group, and the changes experienced by the placebo group, likely due to high variation across participants.
However, the treatment group experienced significant reductions in the area under both the glucose and insulin curves. As discussed above, these metrics track blood glucose and blood insulin across time after consumption of a dextrose drink. Both glucose and insulin rise and then fall in response to consumption of the sugar in the drink, forming glucose and insulin curves. Smaller spikes that return to normal sooner result in smaller “area under the curve” and indicate better metabolic health.
Similarly, the treatment group experienced a large increase in their average insulin sensitivity index, while the placebo group experienced a small decrease in this measure.
Lastly, in terms of reproductive hormones, the treatment group experienced decreases in all androgen hormone measures (total and free testosterone and DHEAS) and an increase in their peak progesterone levels. Females with PCOS tend to have elevated androgen hormones and reduced progesterone levels, so these observed changes indicate improvements in reproductive hormone levels.
Percent change in cardiovascular health measures, metabolic health measures, and reproductive hormones after 6 to 8 weeks of supplementation with either 600 mg of d-chiro-inositol or a placebo by participants with polycystic ovary syndrome (PCOS). “No significant difference” means that any differences found were not statistically significantly different. Source: Iuorno et al. (2002)
supplementation with Myo-inositol alone for pcos
Following studies with d-chiro-inositol alone, a group of studies considered supplementation with myo-inositol alone. Costantino et al. (2009) recruited 42 patients with PCOS from the Hospital of Valdagno in Vicenza, Italy.
Participants were randomly assigned to one of two groups:
Treatment Group: consumed 4 grams of myo-inositol + 400 micrograms of folic acid per day
Control Group: consumed 400 micrograms of folic acid per day
Folic acid was included in both groups because it is a common supplement taken by women trying to conceive, regardless of fertility status, to help prevent neural tube defects in the baby if conception occurs.
Both groups consumed their respective supplement(s) for 12 to 16 weeks.
The table below summarizes the changes found after the treatment period. As was observed with d-chiro-inositol supplementation, supplementation with myo-inositol improves blood pressure, total cholesterol, the areas under the glucose and insulin curves, and insulin sensitivity. It also reduces the androgen hormones while increasing progesterone. While the study groups and study duration differ, the magnitudes of the effects found are surprisingly similar across studies.
Percent change in cardiovascular health measures, metabolic health measures, and reproductive hormones after 12 to 16 weeks of supplementation with either 4 grams of myo-inositol + 400 micrograms of folic acid or with 400 micrograms of folic acid alone by participants with polycystic ovary syndrome (PCOS). “No significant difference” means that any differences found were not statistically significantly different. Source: Costantino et al. (2009)
A second study, Artini et al. (2013) recruited 50 females with PCOS among patients for Assisted Reproductive Technology services at the University of Pisa Division of Obsetrics and Gynaecology. The participants were randomly assigned to one of two groups:
The treatment group: consumed 2 grams of myo-inositol + 200 micrograms of folic acid dissolved in water and consumed in the morning
The control group: consumed 400 micrograms of folic acid daily (delivery mechanism is unclear from the paper)
Both groups followed their respective supplement plan for 12 weeks. It should be noted that this study uses half the dose of myo-inositol as the previously discussed study.
The table below shows the effects of supplementation on reproductive hormones and markers of metabolic health. The group supplementing with only folic acid saw no changes in any of these markers. Note, folic acid supplementation in women trying to conceive and pregnant women is recommended to prevent neural tube defects, which none of these markers predict. So please don’t discontinue folic acid supplementation if recommended by your doctor!
The group that supplemented with both myo-inositol and folic acid saw reductions in luteinizing hormone (LH) and the ratio of LH to follicle stimulating hormone (FSH). In females with PCOS, LH tends to be high, especially relative to FSH, and this imbalance contributes to difficulty ovulating. Reducing LH alone and LH relative to FSH is a positive change.
The other major results include a 52% reduction in fasting insulin levels and a 56% reduction in HOMA-IR, a measure of insulin resistance. These changes suggest improved metabolic health and reduction in risk for type 2 diabetes as well as cardiovascular disease.
Unfortunately, this study did not consider many of the metrics considered by the studies above, so we cannot compare across studies and doses of myo-inositol.
Percent change in reproductive hormones and metabolic health markers after 12 weeks of supplementation with either myo-inositol + folic acid or with folic acid alone. “No significant change” means that any differences found were not statistically significantly different than zero. Source: Artini et al. (2013).
The last study that I will discuss here that only considers myo-inositol does not have a control group but instead splits their sample up between those with normal and those with high fasting insulin levels at the start of the intervention (Genazzani et al. 2012). Given myo-inositol’s role in glucose use and transport (and glycogen storage via conversion to d-chiro-inositol), it makes sense that participants might have different responses to supplementation based on their starting insulin sensitivity. We would expect those with elevated fasting insulin to experience larger benefits from supplementation, and indeed, that is what they find.
In their study, 42 overweight or obese females with PCOS consumed 2 grams of myo-inositol + 200 mg of folic acid per day for 8 weeks. Among these participants, 15 had starting fasting insulin levels at or below 12 micro units per milliliter, which is generally accepted as having normal fasting insulin. The remaining 27 had fasting insulin levels exceeding 12 prior to the intervention.
The table below shows the effects of supplementation on reproductive hormones and markers of metabolic health broken down by starting fasting insulin levels. In both groups, supplementation with myo-inositol reduced luteinizing hormone (LH) and improved the ratio of LH to follicle stimulating hormone (FSH). Interestingly, myo-inositol resulted in decreased FSH among those with elevated insulin levels, which is not the direction we want to see. Other studies find no effect of myo-inositol on FSH levels, so it is unclear if this result would be found if the study were repeated or had a larger sample size.
While supplementation reduced testosterone levels for those starting with elevated insulin, it did not change testosterone levels for those starting with normal fasting insulin levels. Similarly, myo-inositol had no effect on fasting glucose or fasting insulin levels for those starting with normal insulin levels, but it did decrease those markers for those starting with elevated fasting glucose and insulin.
These results are interesting to me on a personal level. When I’ve had blood work done, my fasting glucose and insulin levels have always been normal. When I initially tried to come off of hormonal birth control, I relied largely on myo-inositol and d-chiro-inositol supplementation, and it did not work for me. I did not resume a regular cycle, and my PCOS symptoms got out of control. As I’ve talked about in a previous post, I primarily experience insulin resistance during the luteal phase of my cycle, and adjusting my diet during that phase has been much more impactful for me than inositol supplementation. I would love to see another study with a larger sample size to see if these differential effects still hold.
Percent change in reproductive hormones and metabolic health markers after 8 weeks of supplementation with either myo-inositol + folic acid or with folic acid alone. “No significant change” means that any differences found were not statistically significantly different than zero, and “No significant difference” means that any differences found between groups were not statistically significantly different. Source: Genazzani et al. (2012).
combining d-chiro-inositol and myo-inositol: What’s the optimal combination?
Given the success of myo-inositol and d-chiro-inositol on their own, it makes sense that they might work even better in combination. Nordio et al. (2019) tested various ratios of myo-inositol to d-chiro-inositol to determine which ratio yields the biggest changes in various markers in humans (as opposed to previous studies using mice).
This study recruited 56 females with PCOS. Unlike other studies, this study excluded anyone with a BMI of 30 or higher (the obese category).
Participants were randomly assigned to one of 7 treatment groups that varied based on the ratio of myo-inositol to d-chiro-inositol:
Treatment 1- 0:1 ratio (d-chiro-inositol alone)
Treatment 2- 1:3.5 (mostly d-chiro-inositol)
Treatment 3- 2.5:1 (more myo-inositol)
Treatment 4- 5:1 (even more myo-inositol)
Treatment 5- 20:1 (a lot of myo-inositol!)
Treatment 6- 40:1 (mostly myo-inositol)
Treatment 7- 80:1 (almost entirely myo-inositol)
Each treatment group consumed 2 grams of inositol in total each day, with the composition of the supplement determined by their assigned dosage ratio. Supplementation continued daily for 3 months.
In terms of reproductive hormones, substantial differences were observed across treatment groups in terms of peak progesterone levels. Progesterone levels rise after ovulation. In many females with PCOS, a progesterone peak rarely or never occurs because ovulation is absent or infrequent. And even if ovulation occurs, females with PCOS tend to have lower progesterone peaks. Treatment groups 1, 2, and 3 saw no improvement in peak progesterone values across the three months. The largest improvement occurred for the 40:1 group, followed by the 80:1 group, and then 20:1.
In terms of follicle stimulating hormone (FSH), no changes were observed for any treatment group, but reductions in luteinizing hormone (LH) occurred for both the 40:1 and 80:1 groups, with no significant different between the effects found for these ratios.
In terms of testosterone, only ratios of 20:1, 40:1, and 80:1 led to significant reductions from the baseline, with 40:1 leading to the largest reduction. Similarly, these same three groups saw increases in sex hormone binding globulin (SHBG), with the largest increase observed for the 40:1 group. SHBG binds to free testosterone and reduces its androgenic effects.
In terms of HOMA-IR, a marker of insulin resistance, all ratios led to statistically significant reductions in this marker, with no ratio standing out as superior to the others.
From this study, we can see that if we just care about insulin resistance, any ratio is fine. If we are concerned with the whole suite of reproductive hormones, then the 40:1 ratio is our best bet.
take home points
Supplementation with either d-chiro-inositol or myo-inositol may improve blood pressure, triglyceride levels, LH to FSH ratios, testosterone levels, sex hormone binding globulin, and insulin resistance.
If combining myo-inositol and d-chiro-inositol, a 40:1 ratio leads to the greatest improvements in reproductive hormone levels.
While inositol supplementation leads to improvements, on average, in both lean and overweight/obese females with PCOS, fewer improvements may be experienced by females with fasting insulin levels currently in the normal range (<12 micro units per mL).
references
Artini PG, Di Berardino OM, Papini F, Genazzani AD, Simi G, Ruggiero M, and Cela V. 2013. Endocrine and clinical effects of myo-inositol administration in polycystic ovary syndrome. A randomized study. Gynecological Endocrinology 29(4):375-379.
Costantino D, Minozzi G, Minozzi F, and Guaraldo C. 2009. Metabolic and hormonal effects of myo-inositol in women with polycystic ovary syndrome: A double-blind trial. European Review for Medical and Pharmacological Sciences, 13:105-110.
Genazzani AD, Prati A, Santagni S, Ricchieri F, Chierchia E, Rattighieri E, Campedelli A, Simoncini T, and Artini PG. 2012. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecological Endocrinology, 28(12):969-73 https://doi.org/10.3109/09513590.2012.685205.
Iuorno MJ, Jakubowicz DJ, Baillargeon J, Dillon P, Gunn RD, Allan G, and Nestler JE. 2002. Effects of d-chiro-inositol in lean women with the polycystic ovary syndrome. Endocrine Practice, 8(6):417-423.
Nestler JE, Jakubowicz DJ, Reamer P, Gunn RD, and Allan G. 1999. Ovulatory and metabolic effects of d-chiro-inositol in the polycystic ovary syndrome. The New England Journal of Medicine, 340(17):1314 - 1320.
Nordio M, Basciani S, and Camajani E. 2019. The 40:1 myo-inositol/D-chiro-inositol plasma ratio is able to restore ovulation in PCOS patients: Comparison with other ratios. European Review for Medical and Pharmacological Sciences, 23:5512-5521 doi: 10.26355/eurrev_201906_18223
Disclaimer: This post is not intended to diagnose or treat any medical issues. It is intended for informational purposes only. I am not a medical practitioner. Always consult a trusted healthcare provider with any questions you may have about a medical condition or treatment and before starting any new health care regimen.